Contract Packaging
Validations, Cleaning, Packaging, and Sterilization Services for the Medical Device Industry

Pre-Validated Solutions
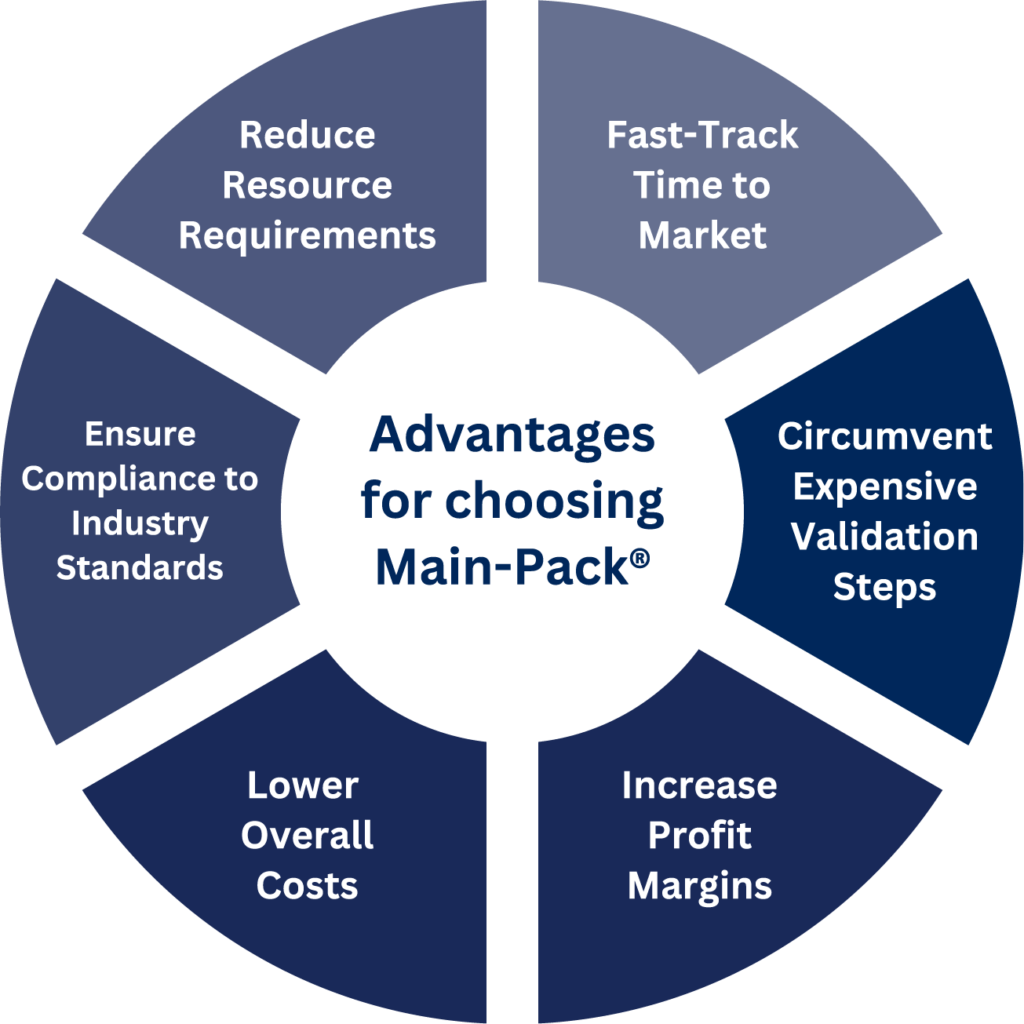
Benefit from Main-Pack®, our family of pre-validated packaging solutions that encompass most medical products. Main-Pack® is designed to minimize validation costs and accelerate time-to-market.

Expert Design and Engineering Support
Our team brings extensive knowledge and proficiency in contract packaging, ensuring precision and reliability in every project.

State-of-the-Art Facilities
Our facilities include two FDA registered clean rooms and controlled environment rooms equipped for both sterile and non-sterile packaging, maintaining the highest standards of cleanliness and control.

Validation Expertise
We understand the concerns many have when switching packaging suppliers, especially regarding validation costs. At Mainstream, we leverage existing validation data from established projects, eliminating the need for costly and time-consuming re-validations. This approach saves you money and accelerates the packaging process.
Packaging Project Development Timeline
Our team will schedule a meeting with you to walk through our processes, learn more about your project, and answer any questions you may have.
We will Share a detailed quote with you that is custom to your project and device. The quote will include all costs from start to finish.
Whether you prefer custom packaging or something off-the-shelf, our experienced packaging engineers will design a solution tailored to your project.
After reviewing and analyzing all of your suppliers’ processes, we will write your custom test protocol, manage your testing, and write your validation reports
We will handle all of your labeling and packaging quality documentation needs, and train our team on your particular device and its cleaning and packaging requirements.
All processes and validations are in place for routine production of your devices.
FAQ
What is Main-Pack®? What does ‘pre-validated’ mean?
Main-Pack® is Mainstream’s pre-validated family of packaging solutions. Unlike other suppliers whose “pre-validated” solutions only apply to a small cluster of medical devices, Main-Pack® encompasses most single-use medical devices. Main-Pack® solutions are “pre-validated” because they have already passed rigorous testing that proves that the packaging will maintain a sterile barrier for a guaranteed period of time. Because this testing has already been completed, when our clients utilize Main-Pack®, we are able to give them their Shelf Life and Selling Process Validations free of charge.
What are your lead times for packaging?
Our lead time for routine production of sterile parts is 3-4 weeks, with non-sterile routine production being 2-3 weeks. This timeframe includes cleaning, packaging, labeling, and sterilization. The lead time for new project development, which includes all tests, validations, document prep, and report writing, is 12-16 weeks. Production and project development timelines can be expedited, if needed.
My product is currently being packaged by a different supplier. How do I move to Mainstream?
Our packaging team is experienced in transferring products to Mainstream. We will review all of your previous validation data and determine which data we can use to make justifications so we don’t have to re-run any unnecessary tests. Depending on justifications and your project’s needs, we can transfer your project to Mainstream in as little time as 6 weeks.
I'm not a packaging expert. How can Mainstream help?
We created Mainstream with the hopes of being the right size supplier for all our clients – there’s no such thing as a silly question here. We will be the packaging experts and guide you through every step of the process so you can focus on launching your project and building your business.